We can describe the state of a thermodymic system uniquely by specifying a small number of macroscopic observables (state variables)
amount of substance (in mol)
Equations of state of the form Form
JOULE's experiment (heat of friction: stirring water with a spindle driven by a weight) proved that heat is a form of energy. Mechanical energy
| (1.1) |
It should be noted that
Internal energy of an ideal gas: The expansion experiment (also by JOULE: expand air from one bottle into an additional, evacuated bottle) demonstrates that
Since
We know even more about
Bringing two bodies in contact such that they may exchange energy, we will in general observe a flow of a certain amount of energy in one direction. As soon as this flow has ebbed off, we have reached thermal equilibrium - by definition.
A measurable quantity to characterize this equilibrium state is the temperature:
Not all transformations of energy that are in accordance with the First Law are really possible. The Second Law tells us which transformations are excluded. There are several logically equivalent formulations:
Impossible are those processes in which nothing happens but a complete transformation ofExample: Expansion of a gas against a weighted piston; here we have a complete change ofinto
. [Kelvin]
Impossible are those processes in which nothing happens but a transport of heatExample: Heat pump; here we do transportfrom a colder to a warmer reservoir. [Clausius]
If a process is such that the inversion of the arrow of time
It is a consequence of the Second Law that there are processes which are not reversible.
Example 1: Reversible expansion
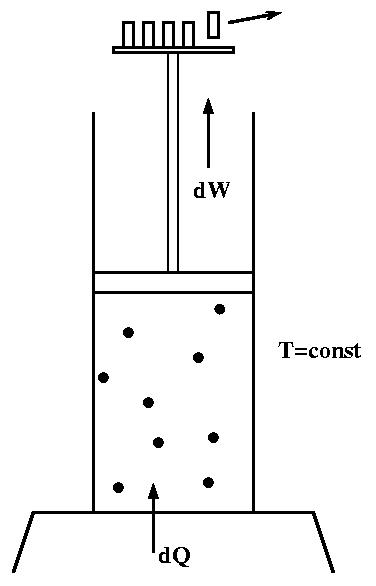
|
Let an ideal gas expand isothermally - i.e. with appropriate input of heat - against a (slowly diminishing!) weight. The internal energy of the gas remains constant,
| (1.2) |
Example 2: Irreversible expansion
Let an ideal gas expand into vacuum (JOULE's expansion experiment). Result:
Changing the direction of time does not produce a possible process: the shrinking of a gas to a smaller volume without input of mechanical energy does not occur.
A mathematically powerful formulation of the Second Law relies on a quantity which is defined as follows:
Let
| (1.3) |
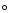
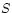 is a state variable, i.e. in any particular
physical state
of a system the entropy
is a state variable, i.e. in any particular
physical state
of a system the entropy 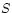 has a well-defined value, independent of
what happened to the system before. This value, however, is defined
only up to an additive constant; the
Third Law removes this ambiguity by stating that
has a well-defined value, independent of
what happened to the system before. This value, however, is defined
only up to an additive constant; the
Third Law removes this ambiguity by stating that
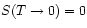 .
.
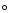
-
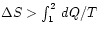 in all irreversible
processes.
in all irreversible
processes.
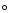
- When a thermally isolated system is in equilibrium the entropy
is at its maximum.
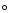
- Thus in a thermally isolated system (the universe being
a notable
special case) we have
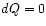 and therefore
and therefore
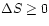 ,
meaning that the entropy will never decrease spontaneously.
,
meaning that the entropy will never decrease spontaneously.
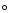
- As a consequence of the First Law
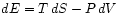 we
have
we
have
But this means that we can describe thermal equilibrium in yet another way: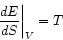
(1.4)
The flow of energy between two bodies in contact will come to an end when
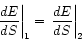
(1.5) 
- If two systems are in thermal equilibrium then
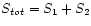 ; entropy is additive (extensive).
; entropy is additive (extensive).
By scrutinizing the entropy balance in a thermodynamic process we may find out whether that process is, according to the Second Law, a possible one. In particular, we can easily decide whether the change of state is reversible or not.
Example 1: Reversible expansion
In the former experiment the entropy of the gas increases by the amount
| (1.6) |
| (1.7) |
Example 2: Irreversible expansion:
Let the initial and final states of the gas be the same as above. Since
| (1.8) |
The thermodynamic state - the ``macrostate'' - of a gas is uniquely determined by two independent state variables. Any of the two mechanical variables -
- *
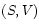 :
The system's volume and heat content are controlled. This may be done
by a thermally insulating the sample (while allowing for controlled
addition of heat,
:
The system's volume and heat content are controlled. This may be done
by a thermally insulating the sample (while allowing for controlled
addition of heat,  ) and manipulating the position of
the piston, i. e. the sample volume
) and manipulating the position of
the piston, i. e. the sample volume  . The internal energy is a (often unknown) function of these variables:
. The internal energy is a (often unknown) function of these variables: 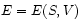 .
.
Now let the macrostate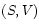 be changed reversibly, meaning that we
introduce small changes
be changed reversibly, meaning that we
introduce small changes 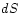 ,
, 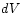 of the state variables. According
to the First Law, the change
of the state variables. According
to the First Law, the change 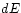 of internal energy must then be the
net sum of the mechanical and thermal contributions:
of internal energy must then be the
net sum of the mechanical and thermal contributions:
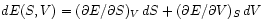 .
Comparing this
to
.
Comparing this
to
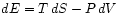 we derive the thermodynamic
(``Maxwell's'') relations
we derive the thermodynamic
(``Maxwell's'') relations
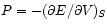 and
and
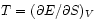 .
.
Special case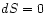 : For adiabatic expansion we see that
the internal energy of the gas changes by
: For adiabatic expansion we see that
the internal energy of the gas changes by
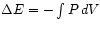 - just the amount of mechanical work
done by the gas.
- just the amount of mechanical work
done by the gas.
The internal energy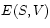 is our first example of a
Thermodynamic Potential. Its usefulness stems from the fact
that the thermodynamic observables
is our first example of a
Thermodynamic Potential. Its usefulness stems from the fact
that the thermodynamic observables 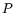 and
and 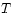 may be represented
as derivatives of
may be represented
as derivatives of 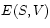 . We will now consider three more
of these potentials, each pertaining to another choice of
state variables.
. We will now consider three more
of these potentials, each pertaining to another choice of
state variables.
- *
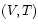 : The system's volume and its temperature are
controlled.
: The system's volume and its temperature are
controlled.
The appropriate thermodynamic potential is then the Helmholtz Free Energy defined by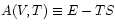 . Using
. Using
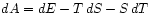
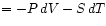 we may derive the relations
we may derive the relations
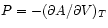 and
and
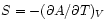 .
.
Special case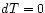 : Considering a process of isothermal
expansion we find that now the free energy of the gas changes by
: Considering a process of isothermal
expansion we find that now the free energy of the gas changes by
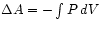 - again, the amount of mechanical work
done by the gas.
- again, the amount of mechanical work
done by the gas.
This is worth remembering: the mechanical work done by a substance (not just an ideal gas!) that is allowed to expand isothermally and reversibly from volume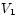 to
to 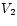 is equal to the difference between the free energies
of the final and initial states, respectively.
is equal to the difference between the free energies
of the final and initial states, respectively.
- *
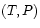 : Heat bath and force-controlled piston.
: Heat bath and force-controlled piston.
Gibbs Potential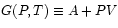 :
:
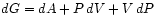
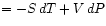
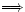
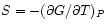 and
and
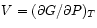 .
.
Special case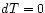 :
The basic experiment described earlier, isothermal expansion
against a controlled force (= load on the piston) is best
discussed in terms of
:
The basic experiment described earlier, isothermal expansion
against a controlled force (= load on the piston) is best
discussed in terms of 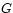 : the change
: the change 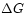 equals simply
equals simply
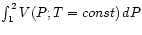 , which may be either measured or calculated
using an appropriate equation of state
, which may be either measured or calculated
using an appropriate equation of state 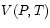 .
.
- *
 : As in the first case, the heat content is controlled;
but now the force acting on the piston (and not the
latter's position) is the controllable mechanical parameter.
: As in the first case, the heat content is controlled;
but now the force acting on the piston (and not the
latter's position) is the controllable mechanical parameter.
Define the Enthalpy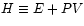 :
:
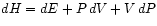
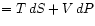
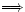
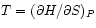 and
and
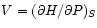 .
.
Special case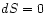 : Again, the system may be allowed to expand, but
without replacement of the exported mechanical energy by the
import of heat (adiabatic expansion). The enthalpy difference
is then just
: Again, the system may be allowed to expand, but
without replacement of the exported mechanical energy by the
import of heat (adiabatic expansion). The enthalpy difference
is then just
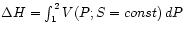 , which may be
measured. (In rare cases, such as the ideal gas, there may be an
equation of state for
, which may be
measured. (In rare cases, such as the ideal gas, there may be an
equation of state for 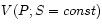 , which allows us to calculate
the integral.)
, which allows us to calculate
the integral.)
The multitude of thermodynamic potentials may seem bewildering; it should not. As mentioned before, they were actually introduced for convenience of description by the ``natural'' independent variables suggested by the experiment - or by the machine design: we should not forget that the early thermodynamicists were out to construct thermomechanical machines.
There is a extremely useful and simple mnemonic scheme, due to Maxwell, that allows us to reproduce the basic thermodynamic relations without recourse to longish derivations: Maxwell's square. Here it is:
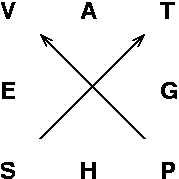
|
||
|
Thermodynamics in a square: Maxwell's mnemonic device |
||
![$\textstyle \parbox{381pt}{
{\large \bf That thing called entropy:} *[12pt]
We...
...E/\partial V)_{S}=-P$ etc. to build up thermodynamics from
first principles!
}$](img168.png)